Mobile Phone Battery Definition and Type
A rechargeable energy storage unit used primarily to supply power to mobile phones. It consists of one or more electrochemical cells and is designed to convert stored chemical energy into electrical energy, allowing the phone to function without being connected to a power outlet. Common types of mobile phone batteries include Lithium-ion (Li-ion), Lithium-polymer (Li-Po), Nickel-Cadmium (Ni-Cd), and Nickel-Metal Hydride (Ni-MH). Among these, Li-ion and Li-Po are the most commonly used in modern smartphones due to their high energy density, low self-discharge, and absence of the memory effect.
This battery enables users to operate their phones for hours or days, depending on their usage and the battery's capacity. Over time, with repeated charging and discharging cycles, the efficiency and capacity of the battery may decrease, leading to reduced phone usage time before a recharge is needed.

Mobile Phone Battery Type
Mobile phone batteries have evolved over the years, and the type of battery used in a mobile phone largely depends on the era and technology available at the time. Here are the primary battery types that have been, or are still used in mobile phones:
1. Nickel-Cadmium (Ni-Cd)
An early rechargeable battery type. It was relatively heavy and suffered from the "memory effect," where the battery could lose its maximum energy capacity if repeatedly recharged without being fully discharged.
♦Advantages:
Durable: It can withstand high discharge rates and is less susceptible to damage from overcharging.
Cheaper: Generally less expensive than Li-ion and Li-Po.
♦Disadvantages:
Memory Effect: It needs to be fully discharged before recharging to maintain capacity.
Lower Energy Density: Holds less energy compared to Li-ion and Li-Po.
Environmental Concerns: Cadmium is toxic and requires special recycling processes.
-battery.webp)
2. Nickel-Metal Hydride (Ni-MH)
An improvement over Ni-Cd batteries. They have a higher energy density and a reduced memory effect. Still, they're heavier compared to modern batteries and have a faster self-discharge rate.
♦Advantages:
Higher Energy Density: Holds more energy than Ni-Cd.
Less Memory Effect: Less pronounced than in Ni-Cd batteries.
Environmentally friendly: Doesn't contain toxic cadmium like Ni-Cd.
♦Disadvantages:
Heavier: Typically heavier than Li-ion and Li-Po batteries.
High Self-Discharge Rate: It can lose its charge relatively quickly when not in use.
Shorter Lifespan: Typically offers fewer charge cycles than Li-ion.
--Battery.webp)
3. Lithium-ion (Li-ion)
The most common type of battery used in today's smartphones. Li-ion batteries have a high energy density, are lightweight, and don't suffer from the memory effect. They can also be rapidly charged and have a slow self-discharge rate.
♦Advantages:
High Energy Density: Holds more energy and can power phones longer between charges.
Low Self-Discharge: Retains its charge better than most other rechargeable batteries.
Fast Charging: It can recharge quickly.
No Memory Effect: Doesn't require a full discharge before recharging.
♦Disadvantages:
Ageing: Can degrade over time, even when not in use.
Safety Concerns: They can overheat, leading to potential risks like fire or explosion if damaged or improperly charged.
Expensive: Tends to be more costly than Ni-Cd or Ni-MH batteries.
-battery.webp)
4. Lithium-polymer (Li-Po)
Li-Po batteries offer similar advantages to Li-ion batteries but can be made thinner and in various shapes. This flexibility has made them increasingly popular in slim and uniquely designed devices.
♦Advantages:
Flexible Form Factor: Can be made in different shapes and sizes.
Lightweight: Typically lighter than Li-ion batteries.
Low Self-Discharge Rate: Similar to Li-ion in retaining charge
♦Disadvantages:
Lower Energy Density: These batteries typically offer less energy than Li-ion batteries.
Shorter Lifespan: Generally has fewer charge cycles than Li-ion.
Safety Concerns: Like Li-ion, it can pose a risk if damaged or improperly charged.
-Battery.webp)
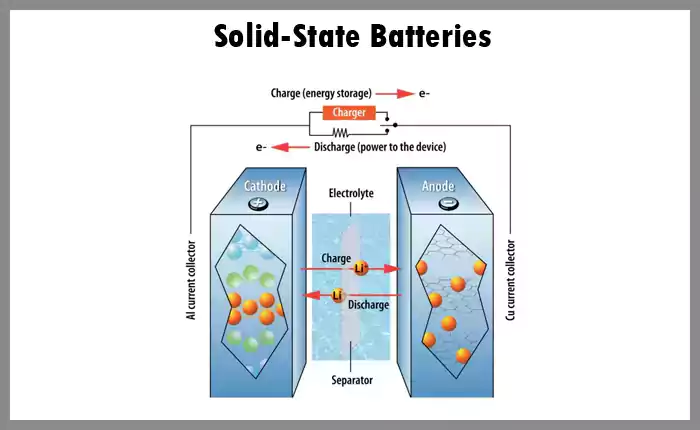
5. Solid-State Batteries
As an emerging technology, solid-state batteries promise higher energy densities, longer lifespans, and improved safety due to the use of solid electrolytes instead of liquid ones. They're not yet mainstream, but advancements are being made in this area.
♦Advantages:
Safety: Solid-state batteries use solid electrolytes, which eliminate the risks of leakage or fire commonly associated with liquid electrolytes.
Energy Density: These batteries typically have a higher energy density than their liquid counterparts. This means they can hold more energy in the same volume, leading to longer-lasting charges for devices and potentially extending the range of electric vehicles.
Longevity: Solid-state batteries generally have longer lifespans and can endure more charge/discharge cycles before their capacity significantly degrades.
♦Disadvantages:
Cost: Currently, the production of solid-state batteries is more expensive than traditional batteries due to technology maturity, production processes, and materials used.
Scalability: Production processes for solid-state batteries at a large scale are still being developed and refined. This makes mass production more challenging at the moment.
Material Challenges: Some solid electrolytes can develop dendrites (tiny, tendril-like structures) over time, which can short-circuit the battery.
6. Lithium Iron Phosphate (LiFePO₄)
Known for their safety and long cycle life, these batteries are less commonly used in smartphones but are found in some other consumer electronics and electric vehicles.
♦Advantages:
Safety: LiFePO₄ batteries are more thermally stable and have a lower risk of thermal runaway compared to other lithium-ion chemistries. This makes them less prone to overheating or catching fire.
Long Cycle Life: LFP batteries often have a longer cycle life than other types of lithium-ion batteries. They can sustain a larger number of charge and discharge cycles before their capacity significantly decreases.
Stable Discharge Voltage: LFP batteries maintain a more consistent voltage as they discharge, which can be beneficial for some applications.
♦Disadvantages:
Lower Energy Density: Compared to other lithium-ion chemistries like Lithium Cobalt Oxide (LiCoO₂) or Lithium Nickel Manganese Cobalt (NMC), LFP batteries typically have a lower energy density. This means they might be bulkier for the same amount of energy storage.
Lower Voltage: LFP cells typically have a nominal voltage of 3.2 V, which is lower than the 3.7V of many other lithium-ion cells. This can affect the design and efficiency of the systems in which they're used.
Weight: While they're safer and more durable, LFP batteries can be heavier than other types of lithium-ion batteries with the same capacity.
-mobile-Battery.webp)
7. Lithium Titanate (Li₄Ti₅O₁₂)
Notably used for its fast recharge capability and long cycle life, it has a lower energy density compared to traditional Li-ion batteries. Less common on mobile phones but used in certain specialty applications.
♦Advantages:
Exceptional Cycle Life: LTO batteries can endure a significantly larger number of charge and discharge cycles than most other lithium-ion batteries. It's not uncommon for LTO batteries to sustain over 10,000 to 20,000 cycles.
Fast Charging: Due to their unique chemistry, LTO batteries can be charged very quickly. Some LTO batteries can be fully charged in less than 30 minutes.
Wide Operating Temperature Range: LTO batteries are known to perform well over a broad range of temperatures, making them suitable for environments with extreme cold or heat.
♦Disadvantages:
Lower Energy Density: LTO batteries generally have a lower energy density compared to other lithium-ion chemistries. This might result in a bulkier and heavier battery for the same energy storage capacity.
Lower Nominal Voltage: LTO cells have a nominal voltage of around 2.4V, which is lower than the more common 3.7 V seen in many other lithium-ion cells. This can affect the design and efficiency of devices in which they're used.
Higher Cost: The production of LTO batteries can be more expensive than other lithium-ion batteries, leading to a higher price point.
Which type of phone battery is best?
The term "best" is subjective and can vary based on the specific requirements of a device or a user. Most smartphones primarily use lithium-ion (Li-ion) or lithium-polymer (Li-Po) batteries due to their distinct advantages. Let's explore the criteria for determining the best battery:
Energy Density:
Lithium-ion batteries generally have a high energy density, meaning they can store a significant amount of energy in a relatively small space, making them ideal for compact devices like smartphones.
Weight and Flexibility:
Lithium-polymer batteries have an advantage when it comes to flexibility in shape. They're also lightweight, which can be a priority in mobile design.
Cycle Life:
All batteries degrade over time, but Li-ion and Li-Po offer a relatively good balance of capacity retention over numerous charge and discharge cycles.
Safety:
While safety concerns with Li-ion batteries have been highlighted in the media due to instances of them catching fire or exploding, such incidents are rare and often relate to manufacturing defects or misuse. Advances in technology and safety measures continue to improve the safety of these batteries.
Cost:
Lithium-ion batteries have been in production for many years, and economies of scale have brought down their cost, making them more affordable for manufacturers and, by extension, consumers.
No Memory Effect:
Unlike older battery technologies, such as Nickel-Cadmium (Ni-Cd), Li-ion and Li-Po batteries do not suffer from the memory effect, meaning they can be recharged without needing to be fully discharged.
Considering these factors, Li-ion is often chosen for its high energy density, long cycle life, and cost-effectiveness, while Li-Po is preferred when a specific shape or lightweight design is essential.
Always remember, that the "best" battery type also depends on individual needs. For instance, someone who prioritizes fast charging might prefer a battery type optimized for that, while someone else might prioritize battery longevity or safety.